Abstract
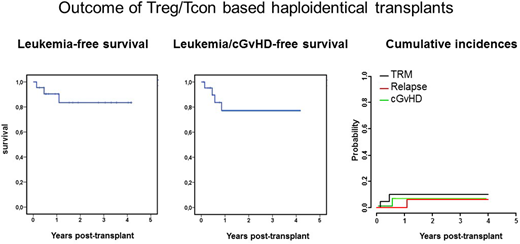
Post-transplant relapse is still a major cause of treatment failure in high-risk acute leukemia (AL) patients. Attempts to manipulate donor T cell alloreactivity to spare normal tissues while killing leukemic cells have been largely unsuccessful. In the search for strategies to separate the GvL effect from GVHD, we investigated the role of a thymic-derived CD4+CD25+ FoxP3+ regulatory T-cell subpopulation (Tregs) that physiologically helps maintain immunological self-tolerance and immune homeostasis. Evidence from murine bone marrow (BM) transplantation across major histocompatibility barriers showed co-infusion of conventional T lymphocytes (Tcons) with Tregs suppressed lethal GVHD without impairing Tcon activity against malignant diseases. In 69 high-risk AL patients who had received an HLA haploidentical T cell-depleted hematopoietic transplant and no post-transplant immunosuppressive GvHD prophylaxis, adoptive immunotherapy with donor Tregs (2 × 106/kg) and Tcons (1 × 106/kg) protected patients from GvHD (Di Ianni et al., Blood 2011) and largely prevented post-transplant leukemia relapse. In fact, only 5% of patients relapsed (Martelli et al., Blood 2014). However, also because such patients had advanced stage disease, TRM was still in the range of that of T cell-depleted haplo transplants, (i.e. 40%, Aversa et al., JCO 2005). A current cohort of AL patients was conditioned with total body irradiation (TBI) (8Gy as single dose TBI or 13.5 Gy as fractionated TBI), cyclophosphamide (30mg/kg), thiotepa (8mg/kg) and fludarabine (200mg/m2). To date, 24 high-risk AL patients (7 ALL, 17 AML) have been enrolled. Twenty-two of the 24 patients engrafted, one AML patient relapsed, 4/22 developed aGvHD (4/4 are alive) and one developed cGvHD, TRM was exceptionally low (Fig. 1). At a median follow up of 2 years, 19 patients are alive. Consequently, probabilities of leukemia-free and leukemia/cGVHD-free survivals are good (Fig. 1). Hypotheses have been put forward to explain the ability of Tregs to prevent of GvHD while preserving the GvL effect mediated by Tcons. However, the mechanism is still unknown. In humans, naïve CD45RA+ Tregs express CXCR4 BM homing receptor and preferentially localize to the BM while memory CD45RO+ Tregs display lower CXCR4 expression and home to the periphery (Booth et al., J Immunol. 2010). Since Tregs that are recovered from peripheral blood and are used for adoptive immunotherapy are CD45RO+ and largely CxCR4 negative, we hypothesized that GvL without GvHD might be due to unopposed Tcon alloreactivity in the BM combined with regulated T cell alloreactivity at the periphery. In order to verify such hypothesis, we infused NSG mice with human leukemia and human Tregs and Tcons. Mice that received leukemia and haploidentical Tcons (without Tregs) cleared leukemia but died of GvHD. T cells harvested from their BM, spleen and liver were predominantly CD8+ and displayed alloreactivity against leukemia. Mice that received leukemia and Tcons plus Tregs were rescued from leukemia and survived without GvHD. T cells harvested from spleen and liver were composed of CD8+ T cells (40%) and CD4+T cells (60%). Purified CD4+ T cells had retained their regulatory function as they inhibited mixed lymphocyte reaction. Purified CD8+ T cells displayed no alloreactivity against leukemia. In contrast, T cells harvested from BM were still predominantly CD8+ and still displayed alloreactivity against leukemia suggesting Tcons had retained their alloantigen recognition. Finally, in mice treated with Tregs alone, T cells were recovered only in the spleen and liver, they displayed a CD4+ phenotype and inhibited mixed lymphocyte reaction. No T cells were found in the BM. In contrast, when we infused human purified CD45RA+ naïve Tregs (that display the CXCR4 BM homing receptor), mice died of leukemia progression despite the co-infusion of large doses of Tcons. In the BM we isolated CD8+ T cells that displayed no ability to kill leukemia and CD4+ T cells that displayed regulatory function. Thus, naïve Tregs homed to the BM and blocked the GvL effect of T cons. When CXCR4 was blocked with an anti-CXCR4 antibody, naïve Tregs could not home to the BM and T cons killed leukemia. In conclusion, Treg-Tcon adoptive immunotherapy confines the graft-versus-host alloreaction to the hematopoietic system and, consequently, allows a GvL effect without GvHD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal